In late 2018, Congress passed the Agriculture Improvement Act, also known as the Farm Bill, changing the federal landscape as it relates to the growth and harvesting of cannabis, or more specifically, hemp. Upon passage of the law, hemp became defined as the Cannabis Sativa L. plant, and any part of that plant, including the seeds, derivatives, extracts, cannabinoids, isomers, acids, etc., with a delta-9 tetrahydrocannabinol (THC) concentration of no more than 0.3 percent by dry weight.1 Hemp meeting that standard was removed from the definition of “marijuana” in the Controlled Substance Act and THC within hemp was given an exemption as a Schedule 1 drug. While hemp varieties of cannabis contain lower THC levels naturally, enough THC may remain in hemp products to produce an intoxicating effect despite meeting the <0.3% standard. As an example, a 30 mL bottle of Cannabidiol (CBD) oil could contain 81 mg of THC and still meet the hemp definition. To put that in perspective, THC levels of 10 mg or higher have been found to cause a “high” in adults, according to studies by Johns Hopkins University.2 So while the Farm Bill was a major shift in the federal stance on the growth and use of the Cannabis Sativa L. plant, the nuances of the law and how it defines the hemp species of the plant are often misunderstood.
On the same day the Farm Bill was passed, the U.S. Food and Drug Administration (FDA) issued a statement to clarify what the legislation meant in relation to medical use of the Cannabis Sativa L. plant and specifically THC-containing products. The FDA statement clarifies that it is illegal to market food or dietary supplements containing added CBD or THC, nor claim any health-related benefit without going through the established FDA drug-approval process. According to the FDA, “selling unapproved products with unsubstantiated therapeutic claims is not only a violation of the law, but also can put patients at risk, as these products have not been proven to be safe or effective.” In short, the statement was a reminder that the FDA will continue regulating products containing cannabis or cannabis-derived compounds at a federal level regardless of whether they are classified as hemp under the Farm Bill.
Thus, with the passage of the Farm Bill, nuances of what the federal government deems “legal” versus what individual states deem “legal” have become even more complex. Further, while the Farm Bill opened the door for the proliferation of “CBD” products that consumers can purchase online or in some cases at a local coffee shop; it did not provide a regulated path to growing, harvesting, producing and distributing cannabis or any cannabis-derived product for medical use. Individual states have established laws to ensure oversight and regulation of the purity of products deemed as “medical marijuana,” but the standards and enforcement of these laws from one state to the next aren’t uniform. Furthermore, state laws do not act as a substitute for FDA approval of the safety profile and efficacy of a drug tested over time, with standardized dosing regimens and consistency of ingredients and manufacturing process.
In November 2019, the FDA issued warning letters to 15 companies for illegally selling products containing CBD in ways that violate the Federal Food, Drug, and Cosmetic Act (FD&C Act) and shared an update with consumers surrounding safety concerns about CBD products. In the statement issued November 25, FDA Principal Deputy Commissioner Amy Abernethy, M.D., Ph.D. noted, “As we work quickly to further clarify our regulatory approach for products containing cannabis and cannabis-derived compounds like CBD, we’ll continue to monitor the marketplace and take action as needed against companies that violate the law in ways that raise a variety of public health concerns. In line with our mission to protect the public, foster innovation, and promote consumer confidence, this overarching approach regarding CBD is the same as the FDA would take for any other substance that we regulate.”3
Without FDA approval of a cannabis product, patients and caregivers take on considerable responsibility to evaluate what they are buying, from whom, how it was grown, and whether it is safe. The reason the FDA drug-approval process exists in the first place is to protect patients, by establishing the safety and efficacy profiles of prescription medicines for specific indications. The reality is most consumers aren’t aware of these nuances in laws or terminology, nor do they have the knowledge or expertise to evaluate the safety of a product, laboratory results or the medical management of a disease.
With the passage of the Farm Bill and the legalization of hemp as defined by the law, the terminology can be confusing, as the plant’s scientific name, legal definitions, molecular contents, and a blurred space in between where the terms (e.g. cannabis, marijuana) are used interchangeably (see Graphic 1 and definitions within the site glossary). The reality is that there are nearly 500 compounds in the cannabis plant13 and to date, the science behind only a handful is well understood. When growing the plant, it is often difficult to know what combination of these hundreds of compounds may be present, even with diligent growing conditions. Below are just a few of the compounds found within Cannabis Sativa L.
Graphic 1: Making Sense of the Terminology
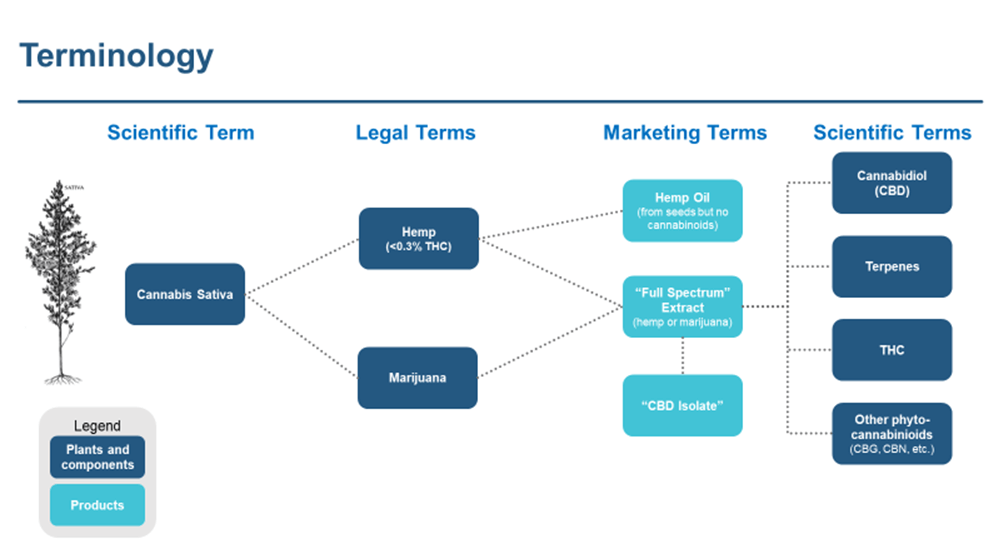
*Definitions available within the Glossary of Cannabinoid Clinical
The recent media “hype” surrounding medical marijuana and the broad availability of CBD-infused products has only led to greater confusion. Medical marijuana products marketed as medication are still illegal under the eyes of the FDA, regardless of what the Farm Bill legalized or individual state legislation. The federal laws and state laws remain in conflict, adding to the confusion and challenges.
The Farm Bill passage was a step forward for cannabis, but it is just that…one step. With progress comes the need for more education and awareness. One year following the passage of the Farm Bill, it is more critical than ever for healthcare providers, policymakers and payers to understand and support the need for cannabis products to adhere to established standards of growing, manufacturing, and distribution in order to ensure patient safety and access to therapies that are shown to be effective in certain patient populations.

References
1Agriculture Improvement Act of 2018. https://www.agriculture.senate.gov/imo/media/doc/Agriculture%20Improvement%20Act%20of%202018.pdf, accessed 11/8/19
2Vandrey R et al, J Analytical Tox, 2017: 1-17
3FDA warns 15 companies for illegally selling various products containing cannabidiol as agency details safety Concerns," FDA News Release, November 25, 2019. Available at https://www.fda.gov/news-events/press-announcements/fda-warns-15-companies-illegally-selling-various-products-containing-cannabidiol-agency-details. Accessed 9 December 2019.
4What Is marijuana? National Institute on Drug Abuse website. https://www.drugabuse.gov/publications/research-reports/ marijuana/what-marijuana Accessed June 20, 2016.
5“Is CBD Legal? Here’s what you need to know according to science.” PBS News Hour, July 12, 2019. Available at: https://www.pbs.org/newshour/science/is-cbd-legal-heres-what-you-need-to-know-according-to-science; accessed 11/8/19.
6Small E, Marcus D. Hemp: a new crop with new uses for North America. In: Janick J, Whipkey A, eds. Trends in New Crops and New Uses. Alexandria, VA: ASHS Press; 2002:284-326. https://www.hort.purdue.edu/newcrop/ncnu02/v5-284.html
7“What is Full Spectrum CBD?” The Street, 6 June 2019. Available at https://www.thestreet.com/lifestyle/what-is-full-spectrum-cbd--14983640. Accessed 8 November 2019.
8“What is CBD Isolate?” The Street, 11 June 2019. Available at https://www.thestreet.com/lifestyle/what-is-cbd-isolate-14986685. Accessed 8 November 2019.
9Brenneisen R. Chemistry and analysis of phytocannabinoids and other cannabis constituents. In: ElSohly MA, ed. Marijuana and the Cannabinoids. Totowa, New Jersey: Humana Press; 2007:17-50. http://www.hampapartiet.se/09.pdf 17.
10Clarke RC, Watson DP. Cannabis and natural cannabis medicines. In: ElSohly MA, ed. Marijuana and the Cannabinoids. Totowa, New Jersey: Humana Press; 2007:1-15. http://www.hampapartiet.se/09.pdf
11Rosenberg EC, Tsien RW, Whalley BJ, Devinsky O. Cannabinoids and epilepsy. Neurotherapeutics. 2015; 12:747-768.http://www.ncbi.nlm.nih.gov/pubmed/?term=7.%09Rosenberg+EC%2C+Tsien+RW%2C+Whalley+BJ%2C+Devinsky+O. +Cannabinoids+and+epilepsy+Neurotherapeutics+2015%3B12%3A747-768/
12Grotenhermen F. Cannabinoids and the endocannabinoid system. Cannabinoids. 2006; 1:10-14. https://www.cannabis-med. org/data/pdf/en_2006_01_2.pdf
13Learn About Marijuana Fact Sheet, Alcohol & Drug Abuse Institute, University of Washington, Available at: https://adai.uw.edu/marijuana/factsheets/cannabinoids.htm; accessed 11/8/19.

